
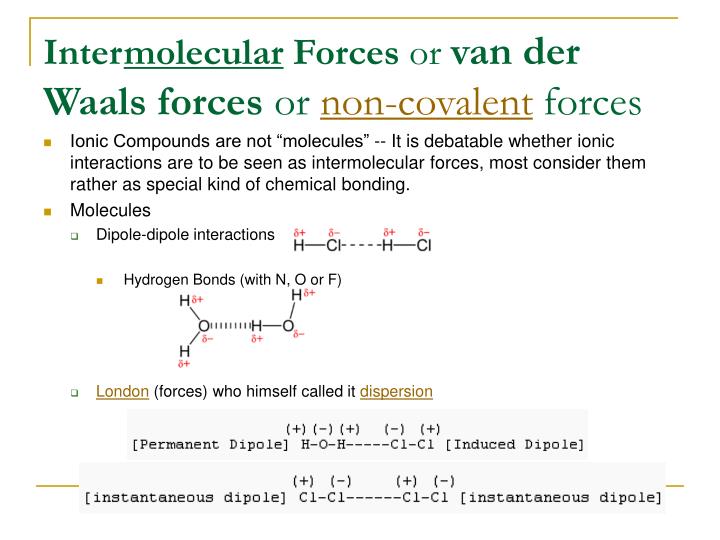
And the area wherein electron concentration is insufficient gets a positive pole. Therefore, at any given instant, the molecule can have a situation where most electrons concentrate on one side, creating a temporary negative pole. In an H 2 molecule, there is constant motion and rapid fluctuations in the electron's positions. The attraction is made possible by the London Dispersion Forces, which later allows the compression of H 2 into hydrogen cylinders. Neutral H 2 molecules can only associate by forming close dimers of Hydrogen gas (H 2) 2. The simplest example of London dispersion force is hydrogen (H 2), a homonuclear diatomic nonpolar molecule. London Dispersion Forces in nonpolar molecules Similarly, neutral atoms like He, Ne, Ar, and butane can also be compressed and liquefied to be sold as Helium/Argon/ Nitrogen or as LPG gas cylinders for home and commercial purposes. For example, in the case of liquefaction of H 2, the gas must be cooled to a very low temperature of -252.87 oC, which slows down its kinetic energy and converts it to a liquid state. Under such conditions, the molecule's kinetic energy decreases, reducing its mobility. The application of vacuum highly favours close atomic contact and liquefaction after that. The conversions are usually done under a vacuum condition that discourages any interference from the air or other solvents. The attractive interaction due to the London Dispersion Forces brings otherwise isolated atoms, and molecules close so that they change state from gas to liquid to solid on the application of lower temperature and high pressure, a process known as liquefaction.

So, there will be no separation of negative and positive poles. These molecules don’t have any interfering functional groups or innate dipoles, making such molecules essentially nonpolar. A few examples of nonpolar atoms or molecules are- CH 4, H 2, I 2, hydrocarbon molecules (alkanes, alkenes, alkynes), and noble gas atoms (He, Ne, Ar). Therefore, to study the London Dispersion Forces in isolation, hydrocarbons, homonuclear molecules, or inert gases are used. Most molecules contain the London dispersion forces in combination with the other prominent forces (dipole, ionic, etc.). It is classified under the three types of Vander Waals forces others are Debye and Keesom forces. The London dispersion force is the smallest yet most significant attractive force common to all atoms and molecules. The shifting electrons’ position in the outer nuclear region creates uncertainty, leading to an attractive universal force, the London dispersion force. The ions have the highest charges, but in uncharged atoms or molecules, charges originate from the constant fluctuations of the electrons. Therefore, most interactions between various chemical entities are controlled by charges.
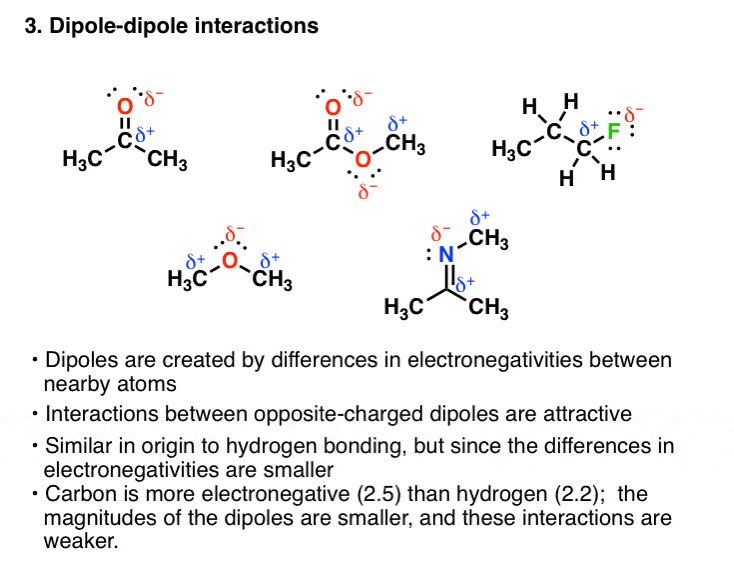
Repulsion occurs when charges are the same.
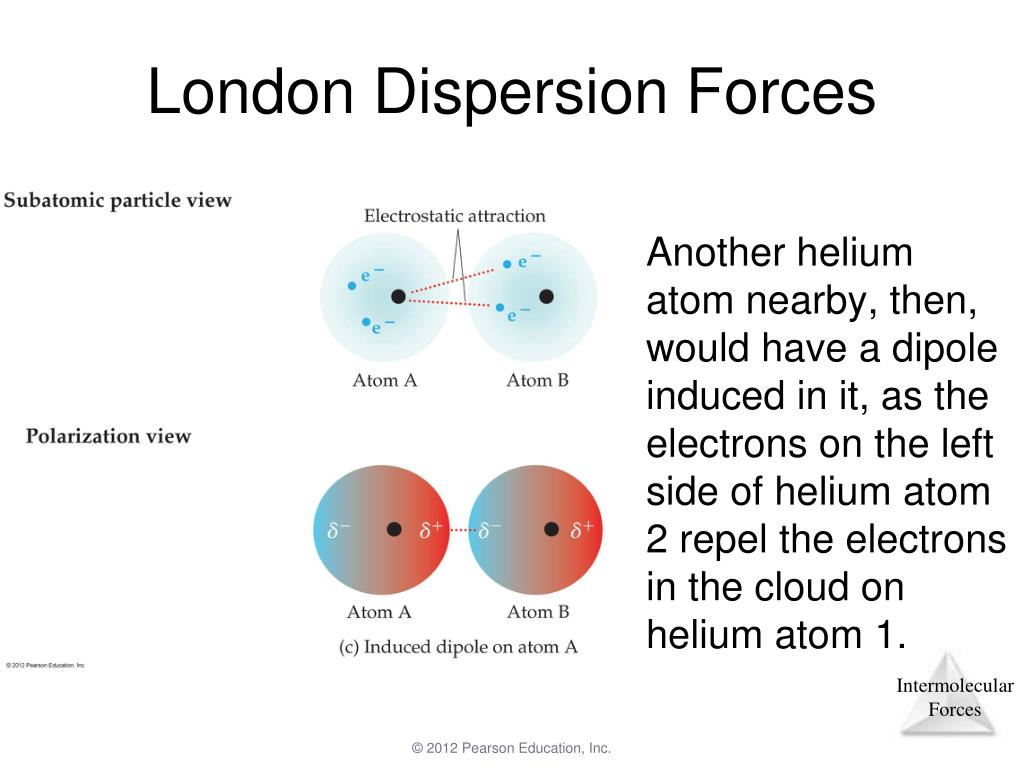
Attraction occurs when the distance between two particles decreases, and they carry opposite charges. The most common way for atoms, molecules, or ions to interact is by attraction or repulsion.


 0 kommentar(er)
0 kommentar(er)
